Semi-Quantitative Cell-Based Indirect Fluorescent Antibody / Semi-Quantitative Indirect Fluorescent Antibody (IFA) / Qualitative Radioimmunoassay (RIA) / Qualitative Immunoblot
Autoimmune dysautonomia encompasses disorders of ganglionic neurons, autonomic nerve fibers, peripheral autonomic synapses, and central autonomic pathways; these disorders are associated with antineural antibodies. Detection of antineural antibodies may help to establish a diagnosis, inform additional testing, support treatment decisions, and guide the search for an associated malignancy.
Disease Overview
Autoimmune dysautonomias may be paraneoplastic or idiopathic, have a subacute or insidious onset, and may present as generalized pandysautonomia or as a more limited form. Symptoms of dysautonomia include anhidrosis, bladder dysfunction, cardiac arrhythmias, gastrointestinal dysmotility (unexplained weight loss, early satiety, anorexia, nausea, vomiting, constipation, or diarrhea), impaired pupillary light reflex, orthostatic hypotension, and bladder dysfunction. Pandysautonomia typically has a subacute onset and is more severe, whereas the limited form is often milder, exhibiting one of just a few of these symptoms.
For more information about laboratory testing for autoimmune neurologic diseases, refer to the ARUP Consult Autoimmune Neurologic Diseases - Antineural Antibody Testing topic.
Test Description
This serum antineural antibody panel test can be used for the evaluation of patients with idiopathic dysautonomia symptoms or symptoms of gastrointestinal dysmotility, or to investigate idiopathic autonomic symptoms and differentiate autoimmune dysautonomia or gastrointestinal dysmotility from the effects of chemotherapy.
Testing for individual autoantibodies is also available separately.
Antibodies Tested and Methodology
| Autoantibody Markers | Methodology | Individual Autoantibody Test Code |
|---|---|---|
| ANNA-1 (Hu) | IFA, reflex IB, reflex titer | 2007961 |
| CASPR2 Ab, IgG | CBA-IFA, reflex titer | 2009452 |
| CV2 (CRMP-5) Ab, IgG | CBA-IFA, reflex titer | 3016999 |
| DPPX Ab, IgG | CBA-IFA, reflex titer | 3004359 |
| Ganglionic AChR Ab, IgG | RIA | 3003020 |
| LGI1 Ab, IgG | CBA-IFA, reflex titer | 2009456 |
| Ab, antibody; AChR, acetylcholine receptor; ANNA-1, antineuronal nuclear antibody type 1; CASPR2, contactin-associated protein 2; CBA, cell-binding assay/cell-based assay; CRMP-5, collapsin response-mediator protein 5; DPPX, dipeptidyl-aminopeptidase-like protein 6; IB, immunoblot; IFA, indirect immunofluorescence assay; IgG, immunoglobulin G; LGI1, leucine-rich, glioma-inactivated protein 1; RIA, radioimmunoassay | ||
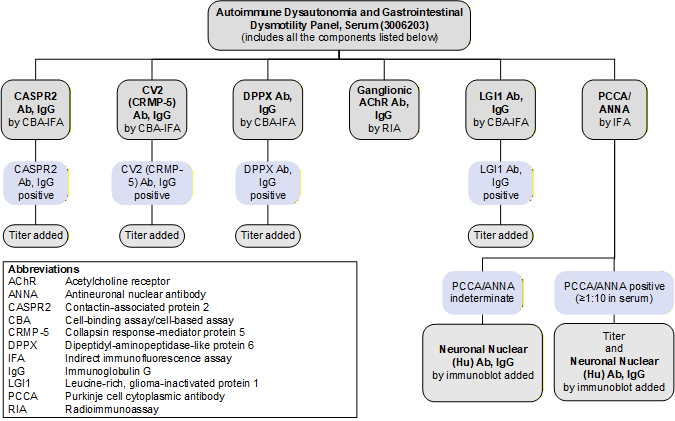
Reflex Pattern
Autoimmune Dysautonomia and Gastrointestinal Dysmotility Panel, Serum (3006203): Reflex Pattern
Limitations
This panel does not include every antibody that has been associated with autoimmune dysautonomia or gastrointestinal dysmotility:
- PCCA-2 is not included because it is extremely rare (present in approximately 0.0001% of specimens submitted for evaluation using a paraneoplastic antibody panel), and commercial assays to confirm the specificity of this antibody are not currently available.
- Adaptor protein 3, subunit B2 (AP3B2) antibody is not included because it has been only recently identified and has been reported in <0.002% of samples screened.
- As testing for newly described antibodies becomes available and their clinical relevance is established, these panels will evolve to reflect these discoveries.
Test Interpretation
Results
Test results must be interpreted in the clinical context of the individual patient; test results (positive or negative) should not supersede clinical judgment.
| Result | Interpretation |
|---|---|
| Positive for ≥1 autoantibodies | Autoantibody(ies) detected May support a clinical diagnosis of autoimmune dysautonomia Consider a focused search for malignancy based on established antibody-tumor associations |
| Negative | No autoantibodies detected A diagnosis of autoimmune dysautonomia or gastrointestinal dysmotility is not excluded |
References
-
30418540
Zalewski P, Słomko J, Zawadka-Kunikowska M. Autonomic dysfunction and chronic disease. Br Med Bull. 2018;128(1):61-74.
-
24833664
Horta ES, Lennon VA, Lachance DH, et al. Neural autoantibody clusters aid diagnosis of cancer. Clin Cancer Res. 2014;20(14):3862-3869.
-
31371564
Honorat JA, Lopez-Chiriboga AS, Kryzer TJ, et al. Autoimmune gait disturbance accompanying adaptor protein-3B2-IgG. Neurology. 2019;93(10):e954-e963.

 Feedback
Feedback
Use to evaluate idiopathic dysautonomia symptoms and gastrointestinal dysmotility symptoms or to differentiate between autoimmune dysautonomia or gastrointestinal dysmotility and the effects of chemotherapy in individuals with these symptoms who are receiving cancer treatment.