Semi-Quantitative Cell-Based Indirect Fluorescent Antibody/Semi-Quantitative Indirect Fluorescent Antibody (IFA)/Semi-Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
Semi-Quantitative Cell-Based Indirect Fluorescent Antibody/Semi-Quantitative Indirect Fluorescent Antibody (IFA)/Semi-Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
Pediatric patients are susceptible to many of the same autoimmune syndromes that affect the central nervous system (CNS) of adults. However, the incidence and prevalence of specific antineural antibodies differ between pediatric and adult patients. In addition, diagnosis in children can be complicated by clinical overlap with other diseases (including genetic, infectious, metabolic, and psychiatric conditions), challenges in obtaining symptom history from very young patients, and the complexities of normal behavior changes during adolescence. Evaluation for the presence of antineural antibodies facilitates treatment, prognosis, and appropriate cancer screening.
Disease Overview
Pediatric patients may develop autoimmune syndromes affecting the CNS that are characterized by diverse phenotypes including behavior changes, disseminated encephalomyelitis, encephalopathy, and epilepsy. Early recognition of possible autoimmune causes of neurologic changes in pediatric patients allows for appropriate diagnostic testing, rapid initiation of treatment, and improved outcomes.
For more information about laboratory testing for autoimmune neurologic diseases, refer to the ARUP Consult Autoimmune Neurologic Diseases - Antineural Antibody Testing topic.
Test Description
These serum and CSF antineural antibody panel tests may be used for the evaluation of pediatric (<18 years of age) patients with subacute onset of encephalopathy, epilepsy, behavior changes, or movement disorders. Testing for the presence of antineural antibodies in both serum and CSF may improve diagnostic yield.
These phenotype-targeted panels test for the presence of antibodies associated with pediatric autoimmune CNS syndromes. Clinical phenotypes for specific antineural antibody-associated syndromes often overlap, and phenotype-specific panels allow for rapid identification of associated antibodies, which may have implications for treatment, prognosis, and cancer screening. For adult patients, other phenotype-specific panels are more appropriate:
| ARUP Panel | Test Code | |
|---|---|---|
| Serum | CSF | |
| Autoimmune Encephalopathy/Dementia Panel | 3006201 | 3006202 |
| Autoimmune Epilepsy Panel | 3006204 | 3006205 |
| Autoimmune Movement Disorder Panel | 3018964 | 3018966 |
| Autoimmune Myelopathy Panel | 3006208 | 3006209 |
Regardless of the panel chosen, order only one panel for serum and/or one panel for CSF; many antineural antibodies are redundant between these panels, and choosing based on the predominant phenotype will provide the most meaningful results. To compare these panels and the antibodies included, refer to the ARUP Antineural Antibody Testing for Autoimmune Neurologic Disease page.
Testing for individual antibodies is also available separately.
Antibodies Tested and Methodology
| Autoantibody Markers | Methodology | Individual Autoantibody Test Code | |
|---|---|---|---|
| Serum | CSF | ||
| AMPAR Ab, IgG | CBA-IFA | 3001260 | 3001257 |
| ANNA-1 (Hu) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| AQP4 Ab, IgG | CBA-IFA, reflex titer | 2013320 | 2011699 |
| CASPR2 Ab, IgG | CBA-IFA, reflex titer | 2009452 | 3001986 |
| DPPX Ab, IgG | CBA-IFA, reflex titer | 3004359 | 3004512 |
| GABA-AR Ab, IgG | CBA-IFA, reflex titer | 3006008 | 3006003 |
| GABA-BR Ab, IgG | CBA-IFA, reflex titer | 3001270 | 3001267 |
| GAD65 Ab | ELISA | 2001771 | 3002788 |
| LGI1 Ab, IgG | CBA-IFA, reflex titer | 2009456 | 3001992 |
| mGluR1 Ab, IgG | CBA-IFA, reflex titer | 3006044 | 3006039 |
| MOG Ab, IgG | CBA-IFA, reflex titer | 3001277 | — |
| NMDAR Ab, IgG | CBA-IFA, reflex titer | 2004221 | 2005164 |
| PCCA-Tr/DNER | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| Ab, antibody; AMPAR, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ANNA-1, antinuclear neuronal antibody type 1; AQP4, aquaporin 4; CASPR2, contactin-associated protein 2; CBA, cell-binding assay/cell-based assay; DNER, Delta/notch-like epidermal growth factor-related receptor; DPPX, dipeptidyl-aminopeptidase-like protein 6; ELISA, enzyme-linked immunosorbent assay; GABA-AR, gamma-aminobutyric acid receptor, type A; GABA-BR, gamma-aminobutyric acid receptor, type B; GAD65, glutamic acid decarboxylase 65-kd isoform; IB, immunoblot; IFA, indirect immunofluorescence assay; LGl1, leucine-rich, glioma-inactivated protein 1; mGluR1, metabotropic glutamate receptor 1; MOG, myelin oligodendrocyte glycoprotein; NMDAR, N-methyl-D-aspartate receptor; PCCA-Tr, Purkinje cell cytoplasmic antibody type Tr | |||
Reflex Patterns
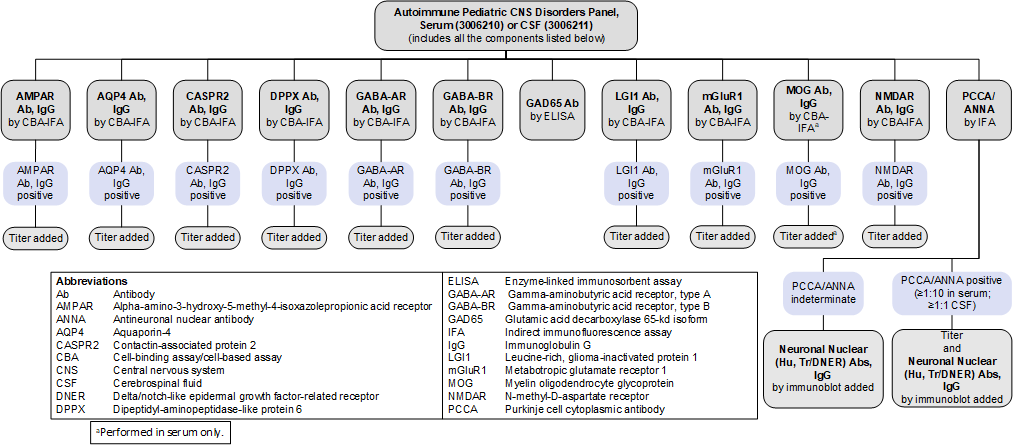
Autoimmune Pediatric CNS Disorders Panel, Serum (3006210) and CSF (3006211): Reflex Patterns
Limitations
These panels do not include every antibody that has been associated with pediatric autoimmune CNS disorders:
- Glial fibrillary acidic protein (GFAP) and neurochondrin are not included in this panel because they have been only recently identified and their prevalence is currently not well established.
- As testing for newly described antibodies becomes available and their clinical relevance is established, these panels will evolve to reflect these discoveries.
Test Interpretation
Results
Results must be interpreted in the clinical context of the individual patient; test results (positive or negative) should not supersede clinical judgment.
| Result | Interpretation |
|---|---|
| Positive for ≥1 autoantibodies | Autoantibody(ies) detected Supports a clinical diagnosis of an autoimmune CNS disorder Consider a focused search for malignancy based on antibody-tumor associations |
| Negative | No autoantibodies detected A diagnosis of an autoimmune CNS disorder is not excluded |
References
-
34955239
Kunchok A, McKeon A, Zekeridou A, et al. Autoimmune/paraneoplastic encephalitis antibody biomarkers: frequency, age, and sex associations. Mayo Clin Proc. 2022;97(3):547-559.
-
31953309
Cellucci T, Van Mater H, Graus F, et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e663.
-
28904568
Barbagallo M, Vitaliti G, Pavone P, et al. Pediatric autoimmune encephalitis. J Pediatr Neurosci. 2017;12(2):130-134.
-
34927485
Wright MA, Trandafir CC, Nelson GR, et al. Diagnosis and management of suspected pediatric autoimmune encephalitis: a comprehensive, multidisciplinary approach and review of literature. J Child Neurol. 2021;37(4):303-313.
-
27112681
Flanagan EP, Drubach DA, Boeve BF. Autoimmune dementia and encephalopathy. Handb Clin Neurology. 2016;133:247-267.
-
29293273
Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166-177.
-
31511329
Shelly S, Kryzer TJ, Komorowski L, et al. Neurochondrin neurological autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e612.


 Feedback
Feedback