Qualitative Immunoblot/Semi-Quantitative Cell-Based Indirect Fluorescent Antibody
Disease Overview
Vision loss may be a symptom of many conditions, including paraneoplastic autoimmune neurologic diseases such as cancer-associated retinopathy and paraneoplastic optic neuropathy. , While these diseases are less common than vision loss due to optic neuritis (as seen in multiple sclerosis and neuromyelitis optica spectrum disorders), including them in the differential for vision loss has important implications for cancer screening and treatment.
For more information about the testing strategy for the following conditions, refer to ARUP Consult topics:
- Multiple Sclerosis
- Neuromyelitis Optica Spectrum Disorders
- Autoimmune Neurologic Disease - Antineural Antibody Testing
Test Description
Consider this phenotype-targeted panel for the evaluation of a subacute onset of progressive bilateral vision loss when there is concern for a paraneoplastic autoimmune etiology.
This panel includes antibodies associated with paraneoplastic vision loss , ; if there is concern for demyelinating disease, consider the Autoimmune CNS Demyelinating Disease Reflexive Panel, which includes AQP4 and MOG antibodies. To compare these panels and the antibodies included, refer to the ARUP Antineural Antibody Testing for Autoimmune Neurologic Disease page. Testing for individual antibodies is also available separately.
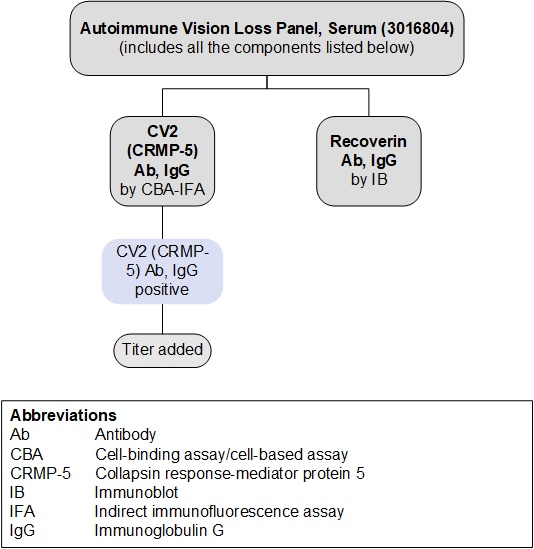
Antibodies Tested and Methodology
| Methodology | Individual Autoantibody Test Code | |
|---|---|---|
| CV2 (CRMP-5) Ab, IgG | CBA-IFA | 3016999 |
| Recoverin Ab, IgG | IB | 3016794 |
| Ab, antibody; CBA, cell-binding assay/cell-based assay; CRMP-5, collapsin response-mediator protein 5; IB, immunoblot; IFA, indirect immunofluorescence assay; IgG, immunoglobulin G | ||
Reflex Patterns
Limitations
This panel does not include every antibody that has been associated with autoimmune vision loss. As testing for newly described antibodies becomes available and their clinical relevance is established, this panel will evolve to reflect these discoveries.
Test Interpretation
Results
Results should be interpreted in the context of the patient’s clinical history, neurologic and ophthalmologic exam, and other laboratory findings. Test results (positive or negative) should not supersede clinical judgment.
| Result | Interpretation |
|---|---|
| Positive for ≥1 autoantibodies | Autoantibody(ies) detected Supports a clinical diagnosis of autoimmune vision loss Consider a focused search for malignancy based on established antibody-tumor associations |
| Negative | No autoantibodies detected A diagnosis of autoimmune vision loss is not excluded |
References
-
27112687
Petzold A, Wong S, Plant GT. Autoimmunity in visual loss. Handb Clin Neurol. 2016;133:353-376.
-
37282453
Wang S, Hou H, Tang Y, et al. An overview on CV2/CRMP5 antibody-associated paraneoplastic neurological syndromes. Neural Regen Res. 2023;18(11):2357-2364.


 Feedback
Feedback