Medical Experts
Couturier

Pertussis, also known as whooping cough, is an acute infectious disease caused by the Bordetella pertussis bacterium. Unlike other vaccine-preventable diseases, pertussis is endemic in the United States, and community transmission remains widespread. In babies and young children, pertussis is especially dangerous and can result in potentially deadly complications, such as pneumonia, apnea, and encephalopathy. Early diagnosis and treatment of pertussis are extremely important to limit disease spread. Recommended laboratory testing for pertussis diagnosis includes culture and polymerase chain reaction (PCR) testing. In addition, although serologic testing is not confirmatory for the purpose of reporting, it may help to identify late-stage pertussis. , Test selection should be based on the amount of time that has elapsed since symptom onset to avoid inaccurate results.
Quick Answers for Clinicians
Early diagnosis and treatment of pertussis (whooping cough) limit its spread to other susceptible people. Laboratory testing enables the collection of accurate surveillance data to assess the impact of pertussis and monitor epidemiologic changes over time. The CDC recommends testing for patients with both high and low suspicion of pertussis for this reason. It is important to note that clinicians should not delay prophylactic treatment for close contacts, particularly infants, pregnant women, and individuals with preexisting health conditions, pending laboratory test results.
A posterior nasopharyngeal swab or aspirate should be obtained from all persons with a suspected case of pertussis (whooping cough). A posterior nasopharyngeal swab is the preferred sample type for adults and adolescents, whereas aspirates are preferable for testing in neonates, infants, and young children. A properly obtained nasopharyngeal swab or aspirate is essential for optimal results. Throat swabs and anterior nasal swabs have an unacceptably low rate of DNA and bacteria recovery and should not be used for pertussis diagnosis. The CDC provides detailed information and instructional videos on best practices for specimen collection on its website.
Indications for Testing
Testing for pertussis is recommended in individuals who have a cough (especially if prolonged) with one or more of the following signs or symptoms suggestive of the disease , , :
- Paroxysms of cough
- Inspiratory whoop
- Posttussive vomiting
- Apnea with or without cyanosis
Additionally, given that paroxysmal cough with an absence of fever is closely linked to pertussis, the absence of fever may further indicate the need for testing.
Laboratory Testing
Laboratory testing is extremely important for the diagnosis and surveillance of pertussis. However, when pertussis is strongly suspected, prophylaxis should be provided to household and other close contacts at high risk without waiting for laboratory confirmation.
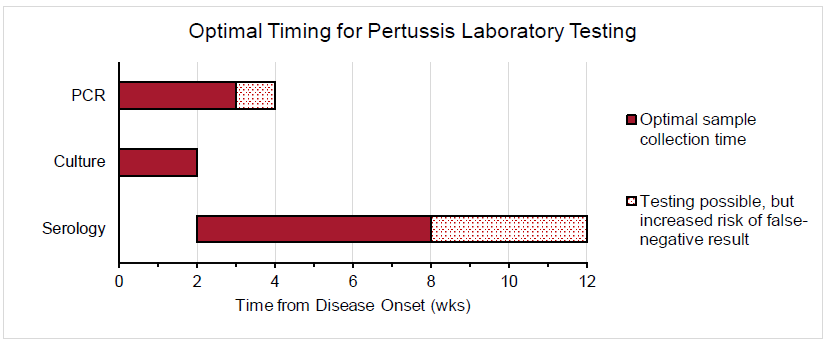
The sensitivity of PCR, culture, and serologic testing is heavily impacted by the time from illness (ie, cough) onset. The figure below depicts the optimal timing for PCR, culture, and serologic testing for pertussis diagnosis.
Polymerase Chain Reaction Testing
PCR is a highly sensitive CDC-recommended laboratory test that is especially useful for the timely diagnosis of pertussis. Specimens for PCR testing should be obtained by aspiration or by swabbing the posterior nasopharynx. Specimen collection for PCR testing should optimally occur within the first 3 weeks of cough onset. After the fourth week of illness, the amount of bacteria in the nasopharynx diminishes quickly, which increases the risk of a false-negative result. Although PCR is a powerful test due to its high sensitivity, the CDC recommends that it be ordered in conjunction with culture when feasible. PCR testing is not recommended for asymptomatic patients because false-positive results are more likely in these individuals.
Culture
Bacterial culture is a highly specific CDC-recommended laboratory test and is considered the gold standard for pertussis diagnosis because it enables strain identification and antimicrobial resistance testing. Patients with suspected pertussis should have a nasopharyngeal swab (specifically noncotton) or aspirate obtained from the posterior nasopharynx to confirm the diagnosis, regardless of concurrent antibiotic use (due to possible antimicrobial resistance). The optimal time to collect a culture specimen is during the first 2 weeks of illness because this is when viable bacteria are present in the nasopharynx. Culture may yield inaccurate results if specimens are collected more than 2 weeks after symptom onset, if patients have received effective antibiotic therapy, or if patients were previously vaccinated against B. pertussis.
Serology
Routine serologic testing is not recommended by the CDC to diagnose pertussis, as recent vaccination, previous infection, and cross-reactivity with other Bordetella species can contribute to inaccurate results. Commercial serology is also not suitable to confirm cases for the purposes of national reporting. However, serologic testing may be useful to assess for late-stage pertussis when the optimal timing for other testing has elapsed. Ideally, specimens for serology should be collected 2-8 weeks after cough onset, when antibody titers are highest. However, serologic testing can be performed for up to 12 weeks after the onset of illness. In patients who were not recently vaccinated, an increasing concentration of immunoglobulin G (IgG) to pertussis toxin or a single titer ≥100 IU/mL can be used to diagnose late-stage pertussis.
ARUP Laboratory Tests
Qualitative Polymerase Chain Reaction (PCR)
Culture
Semi-Quantitative Enzyme-Linked Immunosorbent Assay/Qualitative Immunoblot
Semi-Quantitative Enzyme-Linked Immunosorbent Assay
References
-
CDC - Manual for the Surveillance of Vaccine-Preventable Diseases - Pertussis
Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases, chapter 10: pertussis. Last reviewed May 2020; accessed Sep 2024.
-
CDC - Chapter 22: Manual for the surveillance-bordetella
Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases, chapter 22: laboratory support for surveillance of vaccine-preventable diseases. Last reviewed Mar 2024; accessed Sep 2024.
-
CSTE - Revision to the case definition for national pertussis surveillance
Cieslak PR, Hahn C. Revision to the case definition for national pertussis surveillance. CSTE position statement 19-ID-08 CSTE. Accessed Sep 2024.
-
CDC - Whooping cough-laboratory testing for pertussis
Centers for Disease Control and Prevention. Whooping cough (pertussis): laboratory testing for pertussis. Last reviewed Apr 2024; accessed Sep 2024.
-
30321509
Moore A, Harnden A, Grant CC, et al. Clinically diagnosing pertussis-associated cough in adults and children: CHEST guideline and expert panel report. Chest. 2019;155(1):147-154.


 Cite this page
Cite this page Feedback
Feedback